Who we are
The Mercator Medical Group is a company from Kraków with Polish capital that officially opened its business in the medical devices market in 1996.
It is one of the two companies in Europe and also one of the few companies in the world that distribute both disposable medical supplies and disposable gloves.
The Group is now one of the most important players on the international scene.
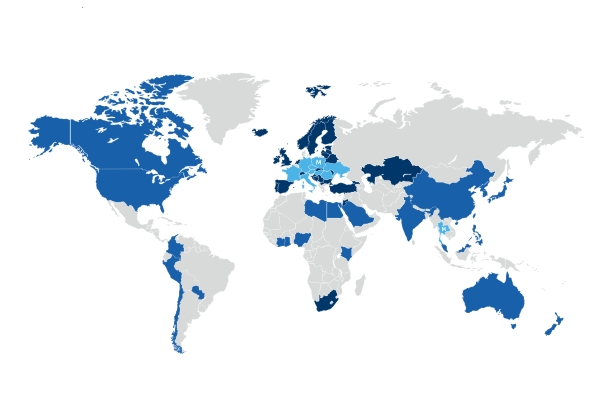
It sells its products to approximately 70 countries in the world and distributes them in Europe.
For over 25 years of its operations, the company has successively established the MERCATOR brand, which is both highly recognisable and well-trusted in the medical industry.
Our portfolio includes a wide range of medical and protective gloves and nonwoven products as well as products from reputable international companies that are distributed exclusively by us.